Are you seeking for 'synthesis of benzocaine mechanism'? You will find questions and answers on the subject here.
Table of contents
- Synthesis of benzocaine mechanism in 2021
- Synthesis of benzocaine lab report
- Synthesis of benzocaine equation
- Benzene to benzocaine
- Synthesis of benzocaine from toluene
- Benzocaine action
- Fischer esterification of benzocaine
- Synthesis of benzocaine experiment
Synthesis of benzocaine mechanism in 2021
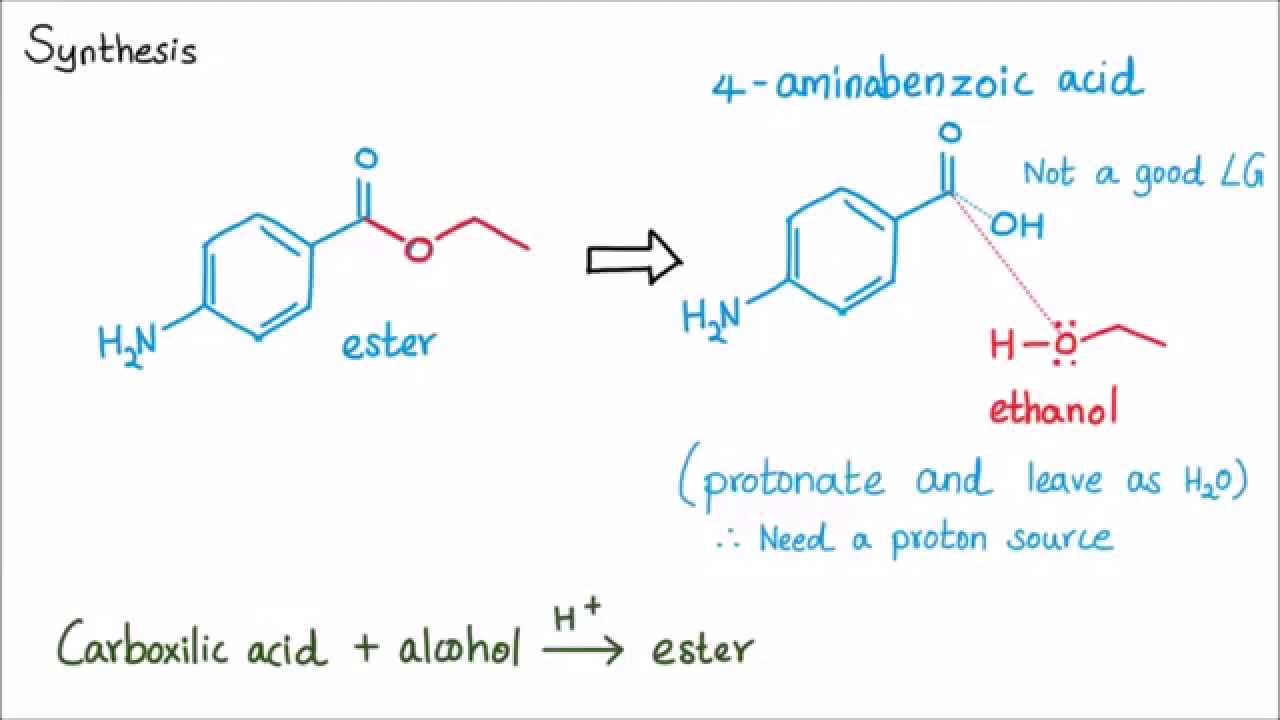 This image shows synthesis of benzocaine mechanism.
This image shows synthesis of benzocaine mechanism.
Synthesis of benzocaine lab report
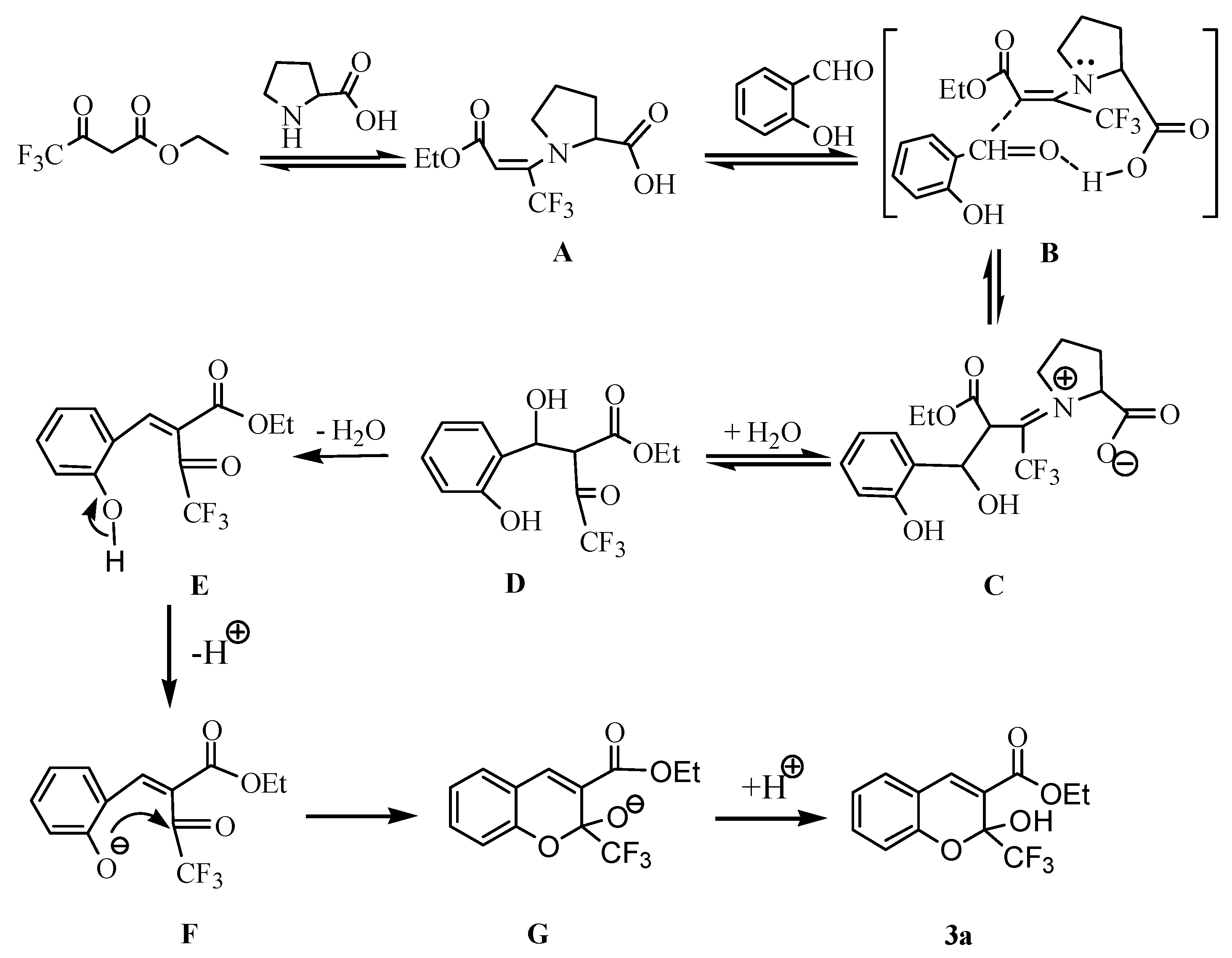 This image shows Synthesis of benzocaine lab report.
This image shows Synthesis of benzocaine lab report.
Synthesis of benzocaine equation
 This image shows Synthesis of benzocaine equation.
This image shows Synthesis of benzocaine equation.
Benzene to benzocaine
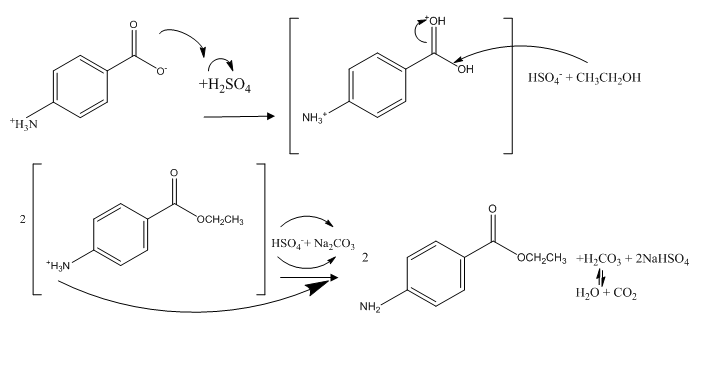 This picture representes Benzene to benzocaine.
This picture representes Benzene to benzocaine.
Synthesis of benzocaine from toluene
 This picture illustrates Synthesis of benzocaine from toluene.
This picture illustrates Synthesis of benzocaine from toluene.
Benzocaine action
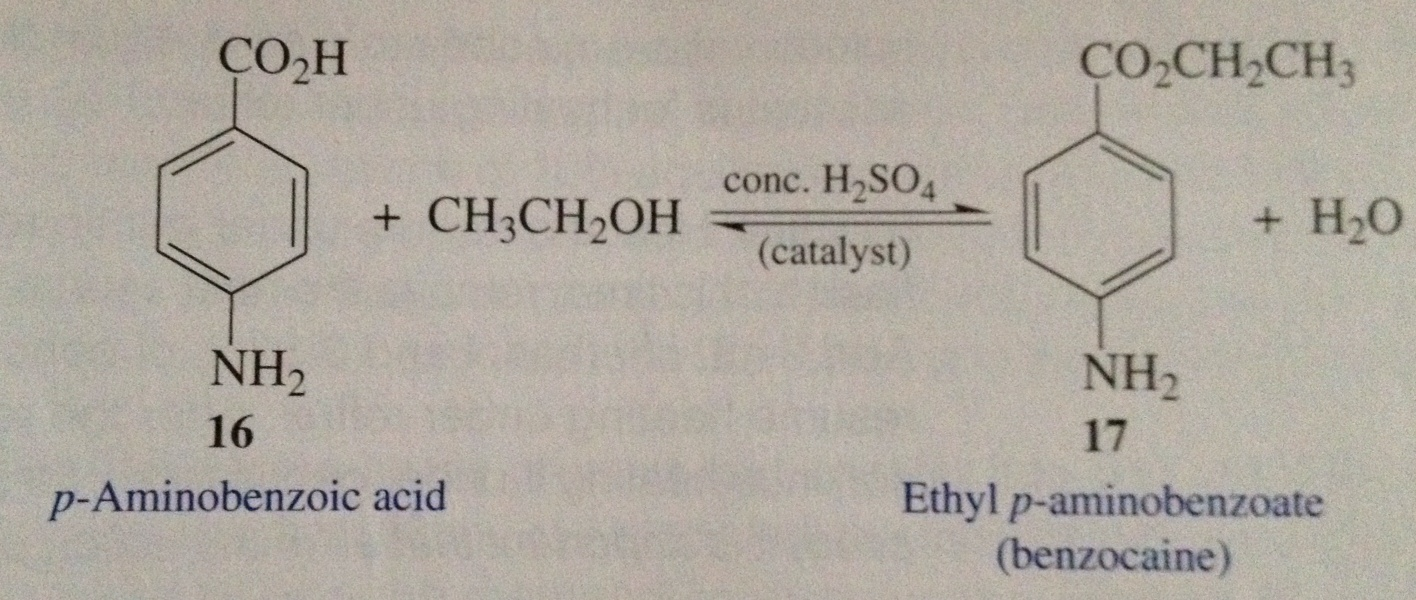 This picture illustrates Benzocaine action.
This picture illustrates Benzocaine action.
Fischer esterification of benzocaine
 This picture illustrates Fischer esterification of benzocaine.
This picture illustrates Fischer esterification of benzocaine.
Synthesis of benzocaine experiment
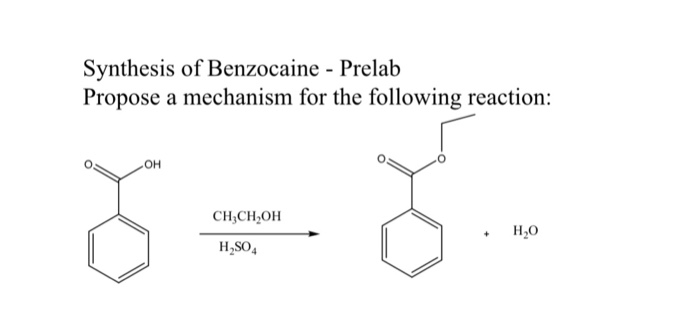 This picture shows Synthesis of benzocaine experiment.
This picture shows Synthesis of benzocaine experiment.
How to make ethyl p-aminobenzoate from benzocaine?
Step 1: Preparation of p-aminobenzoic acid. Place 15 g (0.09 mol) of p-nitrobenzoic acid in a 1-litre round-bottomed flask fitted with a reflux condenser. Introduce 35 g (0.295 mol) of powdered tin and 75 ml of concentrated hydrochloric acid. Heat the mixture gently until the reaction commences, and remove the flame.
What makes benzocaine different from other local anesthetics?
Then after the ether–ethanol layer is dried and evaporated, crystallization of the resulting oil from an ethanol–water solution further purifies the benzocaine. Benzocaine is unlike the other local anesthetics in not having a secondary or tertiary amino group separated from the aromatic portion of the molecule. It
Can a benzocaine be extracted from sodium bisulfate?
Under these conditions, much of the nonionic benzocaine formed in the experiment should remain dis- solved, and some ionic sodium bisulfate and unreacted sodium p–aminobenzoate may precipitate. Extraction of ths mixture with ether permits the separation of the benzocaine from the ionic compounds.
How is benzocaine synthesis used in organic chemistry?
Benzocaine Synthesis I. Introduction Esters are important compounds in organic chemistry. They are used in numerous types ofsynthetic reactions, to create different products for a vast array of purposes, including medicinaland cosmetic. To produce an ester, one can utilize an esterification reaction.
Last Update: Oct 2021