Are you hoping to find 'l pac synthesis'? You can find questions and answers on the topic here.
L-pac produced by biotransformation of benzaldehyde Phenylacetylcarbinol (PAC) is Associate in Nursing organic compound that has two enantiomers, one with R- and one with S-configuration. (R)-PAC, which is commonly known as l-PAC, is renowned as a forerunner in the deductive reasoning of pharmaceuticals much as ephedrine and pseudoephedrine.CAS Number: 1798-60-3Chemical formula: C₉H₁₀O₂Melting point: 9–11 °C (48–52 °F; 282–284 K)Solubility in water: Hopeless
Table of contents
- L pac synthesis in 2021
- Easy ephedrine synthesis
- How to make pseudoephedrine from yeast
- Phenylacetylcarbinol buy
- L-pac to pseudoephedrine
- Other ways to get pseudoephedrine
- Phenylacetylcarbinol synthesis
- L-pac to ephedrine by microwave radiation
L pac synthesis in 2021
 This image representes l pac synthesis.
This image representes l pac synthesis.
Easy ephedrine synthesis
 This picture demonstrates Easy ephedrine synthesis.
This picture demonstrates Easy ephedrine synthesis.
How to make pseudoephedrine from yeast
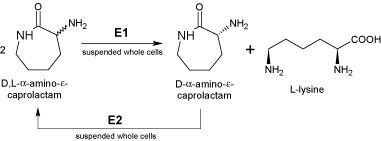 This picture illustrates How to make pseudoephedrine from yeast.
This picture illustrates How to make pseudoephedrine from yeast.
Phenylacetylcarbinol buy
 This image illustrates Phenylacetylcarbinol buy.
This image illustrates Phenylacetylcarbinol buy.
L-pac to pseudoephedrine
 This image shows L-pac to pseudoephedrine.
This image shows L-pac to pseudoephedrine.
Other ways to get pseudoephedrine
 This picture shows Other ways to get pseudoephedrine.
This picture shows Other ways to get pseudoephedrine.
Phenylacetylcarbinol synthesis
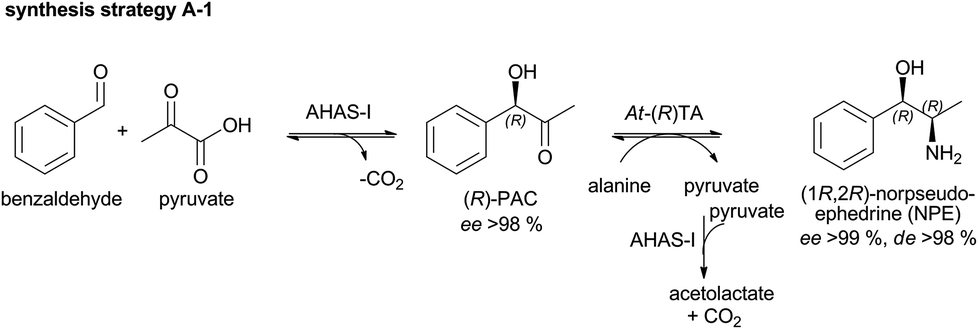 This image illustrates Phenylacetylcarbinol synthesis.
This image illustrates Phenylacetylcarbinol synthesis.
L-pac to ephedrine by microwave radiation
 This image shows L-pac to ephedrine by microwave radiation.
This image shows L-pac to ephedrine by microwave radiation.
Which is a precursor of Phenylacetylcarbinol ( PAC )?
L-pac produced by biotransformation of benzaldehyde Phenylacetylcarbinol (PAC) is an organic compound that has two enantiomers, one with R- and one with S-configuration. (R)-PAC, which is commonly called l-PAC, is known as a precursor in the synthesis of pharmaceuticals such as ephedrine and pseudoephedrine.
How is l-phenylacetylcarbinol prepared in the fermentation process?
L-Phenylacetylcarbinol is a vital intermediate for Ephedrine Hydrochloride and is prepared by the fermentation process using Molasses and yeast (Sachharomyces cerviciae). Benzaldehyde is added at regular intervals when the aldol condensation takes place to give the specific steroisomer.
Which is biochemical reaction condenses pyruvate into r-pac?
There are also biochemical reactions where enzymes such as acetohydroxyacid synthase I from E. coli condense pyruvate and benzaldehyde into R-PAC. Such methods have much higher conversion rates in comparison to the conventional yeast fermentation. ^ "1-Phenyl-1-hydroxypropan-2-one". ^ B, Rosche; V, Sandford; M, Breuer; B, Hauer; P, Rogers (2001).
How is L-PAC produced in the body?
L-PAC is generated biologically through the pyruvate decarboxylase (PDC)-mediated condensation of added benzaldehyde with acetaldehyde generated metabolically from feedstock sugars via pyruvate. Some of the added benzaldehyde is converted through the action of alcohol dehydrogenase(s) to benzyl alcohol, an undesired by-product.
Last Update: Oct 2021